by Stéphanie Peika
The U.S. Food and Drug Administration’s (FDA) Good Manufacturing Practices (GMP) regulations require that an annual review (commonly called annual product reviews) be performed for all drug products. The European GMP regulations and the Canadian GMP guidelines outline corresponding detailed requirements. In each case, there is a general requirement that the quality of each product be reviewed at least once per year “to determine the need for changes in specifications or manufacturing or control procedures” and that any adverse or unexpected trends be identified so that corrective action can be taken. Annual product reviews should encompass a representative number of batches and include considerations of recalls, product complaints, returned and salvaged products, and investigations performed as a result of deviations encountered during production.
Traditionally used in the pharmaceutical industry, annual product reviews can help all auditors define processes, analyze strengths and weaknesses, and find opportunities for improvement. The concept of annual product reviews can be applied to any product manufactured by an organization or any raw material or product received from a supplier. Annual product reviews can also be used as a cost-reduction tool for the auditing process: By conducting annual product reviews, an organization can better control its processes and/or suppliers and reduce the frequency of audits. Annual product reviews can also be used to shorten third-party audits and make better use of audit time.
Annual product reviews
Annual product reviews are becoming part of the pharmaceutical culture around the world. Although their application is currently mostly pharmaceutical-related, other industries can take advantage of the analyses and results obtained from annual product reviews.
Annual product reviews consist of regular periodic or rolling quality reviews of all products. It should be conducted with the objective of verifying the consistency of existing processes, the appropriateness of current specifications for raw materials and finished products, to highlight trends, and to identify product and process improvements. Such reviews should be conducted and documented annually, taking into account previous reviews and product-specific requirements.
As a process, manufacturing a drug is simple and resembles the general manufacturing process of most any product. However, all the steps in the production process yield many opportunities for deviations, changes, and improvements. Annual product reviews will analyze each step, each document, and each decimal point to determine where these improvements can be made based on observed trends and adverse occurrences. Annual product reviews can also be considered process audits, in which all documentation related to a particular process is grouped, analyzed, and trended, and each process is neatly defined and validated.
How annual product reviews can simplify your life and save you money
When performing annual product reviews, a quality overview is essential to cover the basic quality attributes of the process. Annual product reviews should encompass:
- A review of raw materials and packaging materials used for the product, especially those from new sources
- A review of critical in-process controls and finished product testing results
- A review of all batches that failed to meet established specification(s) and their investigation
- A review of all significant deviations, their related investigations, and the effectiveness of resulting corrective and preventive actions
- A review of the results of the stability monitoring program and any adverse trends
- A review of all quality-related returns, complaints, and recalls and the investigations performed at the time
- A review of adequacy of any other previous product process or equipment corrective actions
- The qualification/validation status of relevant equipment and utilities (e.g., HVAC, water, compressed gases, etc.)
- A review of all quality/technical agreements to ensure that they are up to date, adequate, and being followed
Even if the jargon is different, every industry can use a variant of the annual product review process to define and design its system strengths and weaknesses.
Annual product reviews are difficult to implement… at first. Although time-consuming in the beginning and discouraging for junior staff, an experienced quality professional will appreciate the benefits of performing annual product reviews, which include:
- Processes can be charted, allowing non-value-added steps to be identified and processes streamlined
- Trending of deviations can be performed and areas for improvement quickly determined with simple statistical tools
- Trending on rejected batches can be performed, and processes can be better validated/qualified to rectify the most common reasons for rejection
All of this information is then presented in a summary report for each product or process, and this report is reviewed and approved by senior management. Generally, annual product review reports will contain the following information, which is very useful for the auditor:
- An abstract highlighting problems and areas for improvement. The abstract should be short and concise and should include the status of the product review report (process in control, preventive action recommended, or corrective action required).
- A brief discussion of the reason for the annual product reviews and the time period being reviewed, as well as the basic product information (identifying details, e.g., name)
- A discussion of the protocol developed for the annual product reviews, how data were obtained, and how they were analyzed. Also discuss any particular needs that are specific for the product.
- Use tables and graphical representation, where applicable. All steps through which a product goes will be reviewed.
- An analysis of the data, identifying trends that may signal future problems, areas where process control can be employed, and areas where process controls could be tightened. Discuss the need for any change to the process, previous annual product reviews, and corrective action (if any) that were required.
- Recommendations on the improvement of the process or the product, based on the data analyzed
For an auditor, reviewing annual product reviews can save precious time and money. The advantages of annual product reviews are simple:
- A summary report outlines the findings, the trends, and the corrective and preventive action (CAPA) for each product or process.
- Annual product reviews outline the deficiencies in each system.
- Annual product reviews can outline which suppliers or departments should be closely monitored and/or audited. This allows the auditor to focus efforts on the most problematic suppliers (or departments), while continuing to monitor other suppliers and departments for performance.
- Annual product reviews aid in audit preparation and execution. All data are readily available and statistical sampling plans used during the audit can be determined based on the data in the annual product reviews.
Case study
Let’s take the example of OxyPharm, a fictitious pharmaceutical company that manufactures a fictitious allergy medication called SneezeAway, which is a rapid-dissolve tablet. SneezeAway is manufactured using the just-in-time (JIT) production technique.
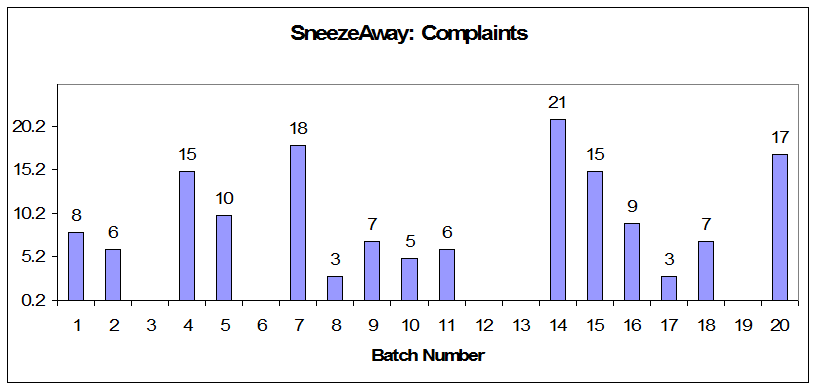
Each year, OxyPharm produces 20 batches of SneezeAway. Of those, an average of five batches are rejected, which costs OxyPharm $25,000 per batch. Last year, one batch of product was recalled due to particles in the bottle. The recall cost $1 million and caused many unhappy clients. Furthermore, each year, the company receives an average 150 complaints on SneezeAway, compared to an average of 50 for each of its other products.
The manufacturing process and documents are audited twice yearly by OxyPharm’s internal auditors, and the following documents are reviewed:
- Statistical sample of manufacturing records
- Statistical sample of testing results
- Statistical sample of retained product (samples kept for testing in case of stability problems, investigations, or for regulatory purposes)
- Listings of product complaints and statistical sampling of complaint investigations and reports
- Listings of deviations and statistical sampling of investigations and reports
- Listings of CAPA and due dates
The auditor must define how many documents of each type he or she must review and to what degree they should be reviewed. Such a system can lead to omissions if all the problems are not picked up in the statistical sampling—especially considering the size of the sample; statistically speaking, one sample out of 20 batches is not much. Case and point: in the five years that OxyPharm has been manufacturing SneezeAway, none of the CAPA or punctual system improvements it’s performed have helped with the rejection rate or the complaint status and the recall couldn’t have been avoided.
If annual product reviews had been performed on SneezeAway for any given year, the main production data could have looked like table 1.
Table 1: Annual product reviews: Simplified results of SneezeAway annual product review
| Batch Number | Final Batch Status | Assay (%) limits: 90-110% | Mixing Time (minutes) | Hardness, SCUlimits: 5-11 SCU | Number of complaints |
| 1 | Released | 101.3 | 5.5 | 8.1 | 8 |
| 2 | Released | 100.2 | 5.4 | 8.2 | 6 |
| 3 | REJECTED | 113.1 | 7.7 | 10.7 | N/AP |
| 4 | Released | 99.7 | 4.8 | 7.9 | 15 |
| 5 | Released | 102.3 | 5.7 | 8.0 | 10 |
| 6 | REJECTED | 89.3 | 3.3 | 5.4 | N/AP |
| 7 | Released | 100.7 | 6.0 | 8.0 | 18 |
| 8 | Released | 99.8 | 5.3 | 7.9 | 3 |
| 9 | Released | 98.6 | 5.1 | 8.3 | 7 |
| 10 | Released | 102.9 | 5.8 | 8.1 | 5 |
| 11 | Released | 100.1 | 6.2 | 7.9 | 6 |
| 12 | REJECTED | 87.4 | 3.2 | 5.2 | N/AP |
| 13 | REJECTED | 85.6 | 3.0 | 5.1 | N/AP |
| 14 | Released | 97.5 | 5.5 | 7.8 | 21 |
| 15 | Released | 102.8 | 6.1 | 7.7 | 15 |
| 16 | Released | 100.0 | 5.2 | 7.9 | 9 |
| 17 | Released | 99.9 | 5.1 | 8.0 | 3 |
| 18 | Released | 101.1 | 5.4 | 8.1 | 7 |
| 19 | REJECTED | 112.3 | 7.5 | 10.6 | N/AP |
| 20 | ReleasedRECALLED | 104.3 | 6.1 | 8.1 | 17 |
Batch 20 was recalled due to particles in the bottle. A subsequent investigation revealed that one of the equipments used for packaging was getting old and metal particle were detaching from the capper. But there were still 17 complaints on the batch before it was recalled, almost a record for SneezeAway.
Graphing this data clearly shows trends, out-of-specification results and areas for improvement, as shown in figures 1 through 4.
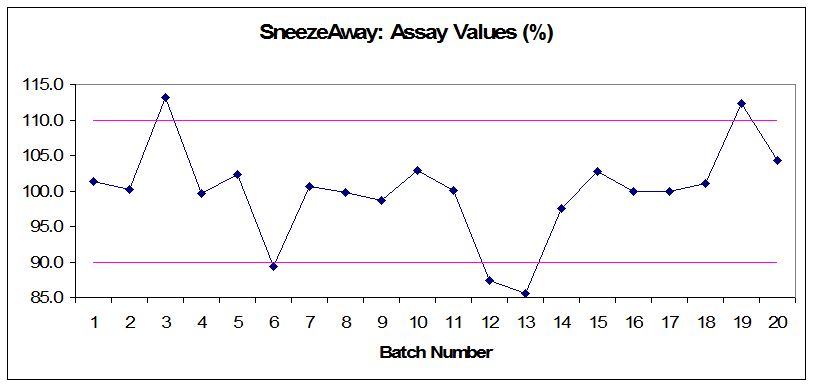
Figure 1: Annual product reviews: SneezeAway assay values (%)
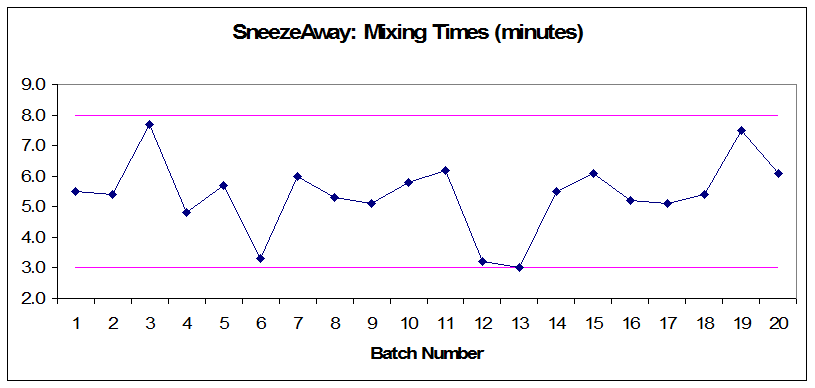
Figure 2: Annual product reviews: SneezeAway mixing times (minutes)
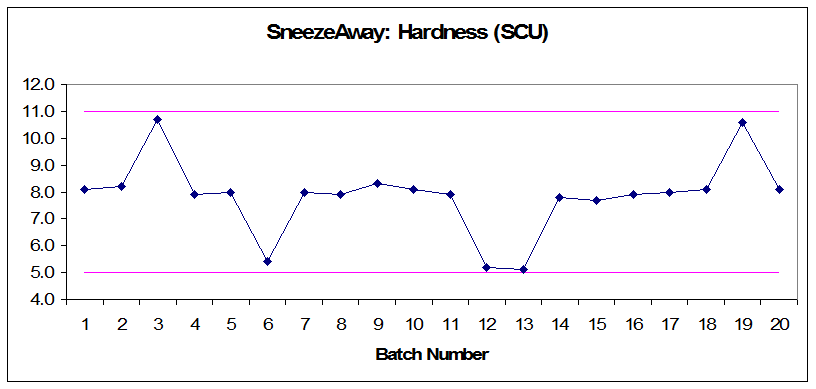
Figure 3: Annual product reviews: SneezeAway hardness (SCU)
Figure 4: Annual product reviews: SneezeAway complaints
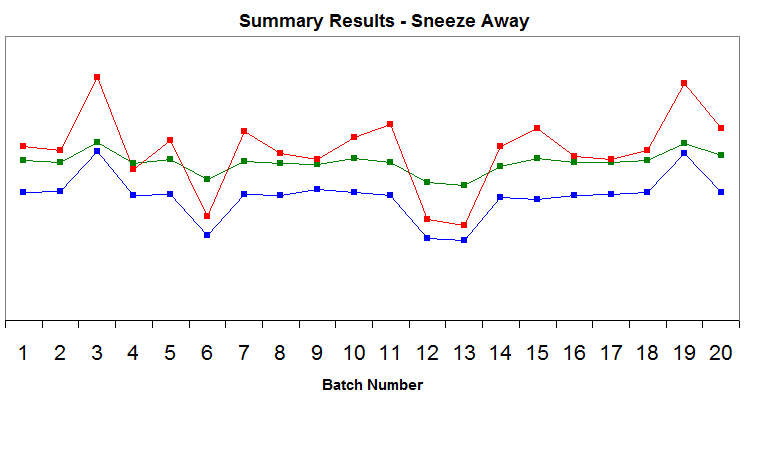
By using the data, graphs, and documents generated by the annual product reviews, OxyPharm’s internal auditors could reduce their auditing times and use it instead on process improvements. They could:
- Determine easily why the rejected batches were rejected (assay values did not meet their pre-established specifications).
- Audit the related documents and make correlations between rejections and process controls. (Rejected batches have mixing times and hardness values that are close to the upper or lower limits, but still within the acceptable range.)
- Review process validation documents and equipment qualification to ensure that the limits set for mixing times and tablet hardness are adequate.
- Assess the need for changes based on the number on complaints on the batches manufactured immediately after the rejected batches.
This process is summarized in figure 5.
Figure 5: Annual product reviews: SneezeAway summary
Economics
Although there is an initial investment required to implement the annual product review process, it allows a company to prepare all the data related to a product, its raw materials, and suppliers. Therefore, once annual product reviews are complete, it’s simple to focus on the most critical suppliers and/or areas for improvement, thus focusing economic efforts in the right directions for an audit.
Economic efforts for the organization can be translated into:
- Process management:
- Better use of resources on process improvements
- Reduced number of rejects
- Reduced number of complaints
- Reduced number of recalls
- Improved equipment performance
- Improved process performance and yields
- Audit management
- Review of agreements in light of annual product review results
- Possible review of purchase prices
- Tightening or loosening of incoming material inspection criteria
- Audit management
- Reduced number of internal and external audits as suppliers and “for cause” audits are better identified
- Reduced travel costs, because suppliers and audit frequencies are better focused
- Shorter audit times, because audits are better prepared and deficient systems and processes are better identified
- Reduced costs of audit samples, because the appropriate processes can be sampled
- Reduced number of auditors (on a corporate level, this is also considered cost saving)
In the OxyPharm example, if the low assay values causing the batch failure were due to:
- A raw material whose test results were close to the lower acceptance limit, which was also observed during the preparation of other annual product reviews with raw materials manufactured by the same supplier, then the OxyPharm auditor would have known that one supplier audit would kill more than one bird with the same stone.
- Mixing times and hardness values being inconsistent, a map of the manufacturing process—prepared during annual product reviews—would identify the parameters for validation. After a process is validated, the need or frequency of audits could be reduced along with the number of rejects
Annual product reviews: Conclusion
As the OxyPharm example demonstrates, annual product reviews allow auditors to get a bird’s-eye-view of the process to be audited and better focus on the areas already defined as being deficient. Most companies operate many processes. Annual product reviews analyze each of them and allow auditors to better define the deficiencies and evaluate the efficacy of corrective and preventive measures.
Annual product reviews provide a glimpse into the past and the future: examining past trends and deviations allows a company to adjust for the future and to strive for process excellence.
About the author
Stéphanie Peika is an ASQ certified quality auditor and a certified manager of quality and operational excellence. She is associate director of quality systems at Paladin Labs Inc., a Montreal-based pharmaceutical company.
Tags: annual product reviews, process auditing.